Unfortunately, the 10-year track record of use of the vaccine, in varying forms, tells us that the claim being made by health authorities that the HPV vaccine is both ‘safe and effective’ is false.
In terms of claimed safety, health authorities are ignoring or seeking to cover-up the scale and severity of the serious and long-term side effects being experienced by adolescent girls. Regarding effectiveness, health authorities are misleading the public by claiming that the vaccine’s ability to raise HPV antibodies over a few years equates to protection from HPV-related cancers, especially cervical cancer, decades down the line. The science tells us that the two are not equivalent.

A Onondaga County, New York (USA) advert promoting the HPV vaccination HSE Ireland HPV vaccine ad campaign
Five Things You Should Know Before Consenting to HPV vaccination
- Be informed before giving consent! Health authorities responsible for national vaccination programs DO NOT offer informed consent. Proper informed consent is about giving a patient whatever information they need to make a decision about proposed medical treatment, including about non-vaccination based approaches. Not what a doctor or nurse thinks they should be told. This should include both risks and benefits, plus alternatives to said treatment. A landmark case on informed consent in the UK means that informed consent should include whatever a patient wants to know, not just what a doctor or nurse thinks they should be told.
To find out more, search ‘HPV vaccine’ on:
- ANH International HPV Vaccine Choice campaign
- ANH-USA Protect Vaccine Choice campaign
- European Medicines database on adverse drug reactions, EudraVigilance. Search for Gardasil, Gardasil 9 and Cervarix.
- Vaccine Adverse Event Reporting System (VAERS) US website. Search for Gardasil, Gardasil 9 and Cervarix.
- HPVvax Film website – soon to be released ANH-USA documentary website
- SaneVax
- National Vaccine Information Center
- Health authorities are misleading the public when they imply that HPV vaccine confers protection against cervical cancer. It’s simply too early to tell how well elevated immunogenicity (i.e. being ‘seropositive’) prevents future cases of cervical cancer. For example, the UK NHS says “The HPV vaccine is effective at stopping girls getting the types of HPV that cause most cervical cancers” without saying that many girls already have HPV before becoming sexually active and most infections resolve on their own through the action of a healthy immune system. Some strains of HPV, like HPV-16, that are associated with cervical cancer, appear to have co-evolved alongside humans and are widely present in girls and women both with and without cervical cancer.
- HPV is largely a transient infection and 90% of HPV infections resolve naturally within 2 years without vaccines or other medical interventions
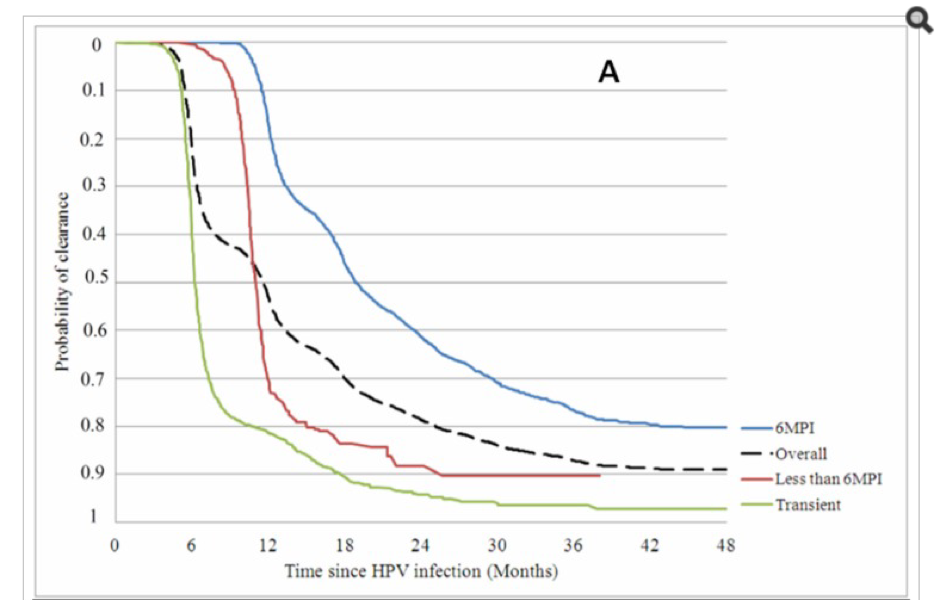
Natural clearance of potentially oncogenic HPV infection in females in the control arm of the PATRICIA HPV vaccine trial. Reference: Jaisamrarn U, et al. Natural History of Progression of HPV Infection to Cervical Lesion or Clearance: Analysis of the Control Arm of the Large, Randomised PATRICIA Study. PLoS One. 2013; 8(11): e79260
- By far the majority (~80%) of cervical cancer incidence and deaths occur in developing countries. This appears to be associated with poor access to screening (Pap smear testing) or early treatment, higher rates of adolescent sexual activity, greater rates of STD infection, lower nutritional status, and other cervical cancer risk factors. Women in Western and industrialised countries are generally not told by health authorities that most of the cervical cancer burden occurs in developing countries.
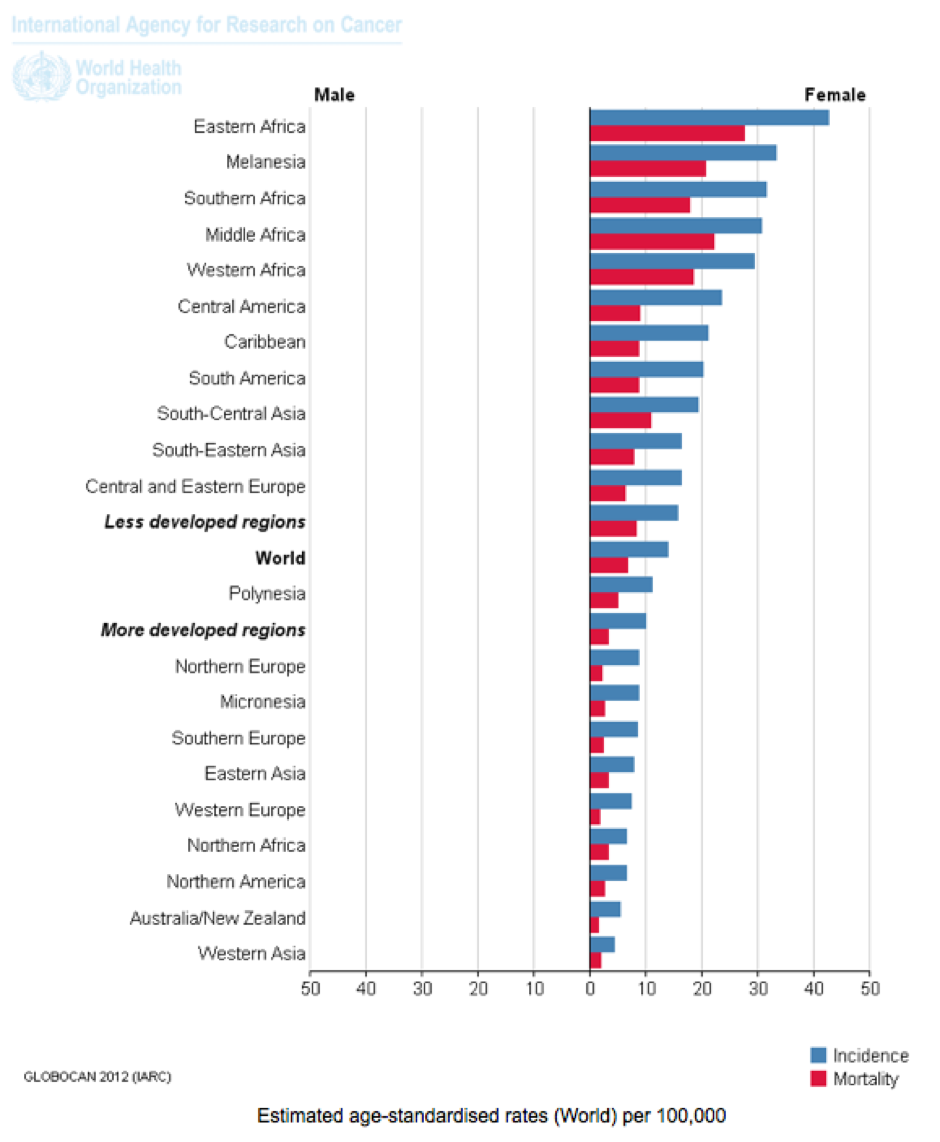 Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Source: IARC, WHO
Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Source: IARC, WHO
- Cervical cancer screening is crucial for early detection of cervical cancer. Women over 25 should be undergoing cervical cancer screening (Pap smear testing) every 3 to 5 years. HPV vaccine doesn’t eliminate the need for Pap smears that should occur more frequently in a woman’s 20s (every 3 years) than in later life (every five years). Owing to a risk of false negatives, as well as natural clearance of HPV or remission, annual testing should follow any detection of positive cytological results (cervical intra-epithelial neoplasia or CIN). Cancer treatment interventions should only be taken following positive results in more than two consecutive tests confirming early microinvasive cervical cancer that can be treated with a high degree of success using targeted techniques such as conisation with laser CO2 excision. Diet and lifestyle should also be modified to minimise future cancer risk.
Article provided by http://anhinternational.org/





























